SILVERPLATING
History
Silver is valued for its brilliant white metallic shine. It is, in fact, the most reflective of all metals. Interestingly, the word ‘silver’ derives from the Latin argentum, which has its roots in the Proto-Indo-European word for ‘shiny’ and ‘white’. Silver is notable for its non-reactivity. It is characterized as a ‘noble metal’, and grouped with other metals, including ruthenium, rhodium, palladium, osmium, iridium, platinum, and gold.[1]
Along with the other noble metals, silver resists corrosion and oxidization in the air. This makes it perfect for crafting items that are built to last even if they are regularly handled and used, since they will not wear down. Silver has the highest electrical and thermal conductivity of all the metals. While this may seem an unimportant fact, these properties make silver the perfect metal to use in electrical devices. For these qualities, silver is highly sought after in the modern world.[2]
The Industrial Revolution brought about huge changes across society, and none more so than in the production of silver items. One of the most radical of these changes was the invention of the silver-plating process, whereby a thin layer of silver could be applied to the surface a cheaper metal. This allowed objects to be created with the appearance of silver, but at a significantly lower cost.
The earliest form of silver plating was Sheffield plate, where thin sheets of silver were fused to a layer or core of base metal of copper. Plated silver is regularly used in flatware [spoons] and hollowware [tableware such as bowls, coffee pots. After the 1840s, the development of electroplating, nickel silver became popular as a base metal and forms an ideal, strong and bright base for silverware.
Nickel silver first became popular as a base metal for silver-plated cutlery and other silverware, notably the electroplated wares called EPNS (electro-plated nickel silver). It is used in zippers, good quality keys, costume jewelry, for making musical instruments (e.g., flutes, clarinets). Nickel silver is so named for its silvery color and has no elemental silver in it until plated.
The Sheffield plating process is not often used today, as after about 1840 it was generally replaced with the electroplating processes. Electroplating tends to produce a "brilliant" surface with a hard color — as it consists of pure rather than sterling silver and is usually deposited more thinly. [3]
original from National museum collection
BRACELET
Designed by: Unknown
Material: Silver-plated metal, garnet, pearls
Size 5,5 x 6,4 cm
Year: 1900
Photo credit: Nationalmuseum
This bracelet made in the beginning of the 2000th century. The bracelet does not have the natural shapes from the art nouveau movement a and it doesn’t have the strict geometrical shapes form the Art deco which makes me think that it was a part of the
The Edwardian era, like the Georgian and Victorian eras before it, derives its name from the reign of an English King, Edward VII (1901-1910).
White metal and white stones took center stage, and though colors were still worn, platinum (along with other white metals), diamonds, and pearls were the darlings of the era. Fine lacy filigree was used during the Edwardian jewelry due to the development of the acetylene and oxygen troche. It was now possible to create intricate designs that was ornate, intricate, and flowery.
re-designed bracelet
RE-DESIGN
The original bracelet from National museum collection is a true representation classical jewelry, it has intricated stone settings and ornamental round curvy shapes. I wanted to create something that was contemporary in its expression. A bracelet that could not have been created during the Edwardian ear but a bracelet that looked like it was made in the 2100 th centenary.
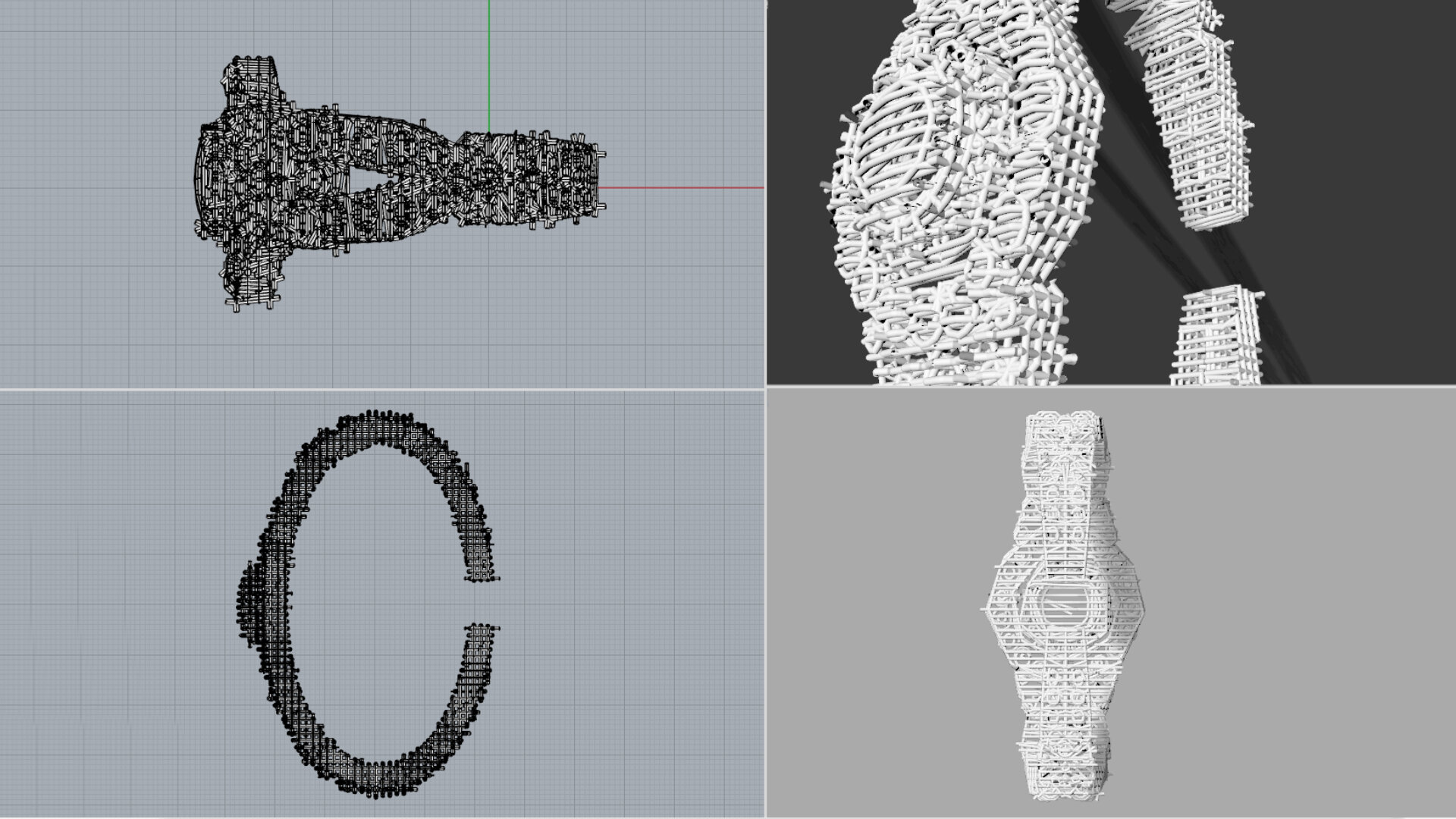
I wanted to use specific qualities that can be found in CAD design. I fist started by making a CAD-drawing of the original design. This drawing was the starting point for the redesigned bracelet. The CAD drawing of the original bracelet contained of 178 individual objects, together the assemble bracelet.
All these 178 parts was deconstructed down to its simplest element, the wire frame the CAD drawing is constructed of. This wireframe it was extracted as network of polylines. The polyline network was then extruded with a 0.8 mm think wire to give the bracelet a body. This created a bracelet that shows the inner workings and structure of a CAD drawing.
process picture
3d-model of redesign
Footnotes
[1] https://www.mayfairgallery.com/blog/history-antique-silver-silverware
[2] https://www.lehigh.edu/~amb4/wbi/kwardlow/conductivity.htm
[3] https://guides.slv.vic.gov.au/hallmarking/silverplating The identification and dating of Sheffield electroplated wares, 1843-1943 by E.R. Matheau-Raven. 1997
This project was made possible with the support of